The recall notification (U.S. only) / field safety notice (International Markets) provides customers with information on how to identify affected products.
Additionally, the device Instructions for Use provide product identification information to assist with this activity.
Products affected by this recall notification (U.S. only) / field safety notice (International Markets) include:
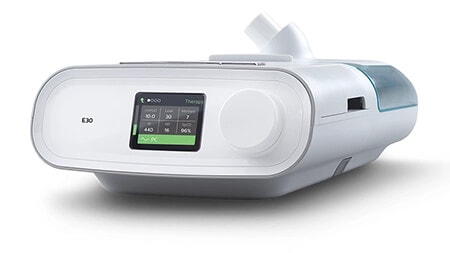
E30
(Emergency Use Authorization)

DreamStation
ASV

DreamStation
ST, AVAPS

SystemOne
ASV4

C Series
ASV, S/T, AVAPS

OmniLab Advanced Plus
In-Lab Titration Device

SystemOne
(Q series)

DreamStation
CPAP, Auto CPAP, BiPAP

DreamStation GO
CPAP, APAP

Dorma 400, 500
CPAP

REMStar SE Auto
CPAP
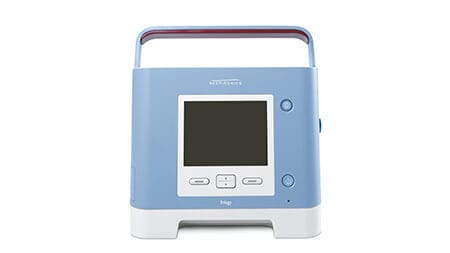
Trilogy 100
Ventilator

Trilogy 200
Ventilator

Garbin Plus, Aeris, LifeVent
Ventilator

A-Series BiPAP Hybrid A30
(not marketed in US)

A-Series BiPAP V30 Auto
Ventilator

A-Series BiPAP A40
(not marketed in US)

A-Series BiPAP A30
(not marketed in US)
